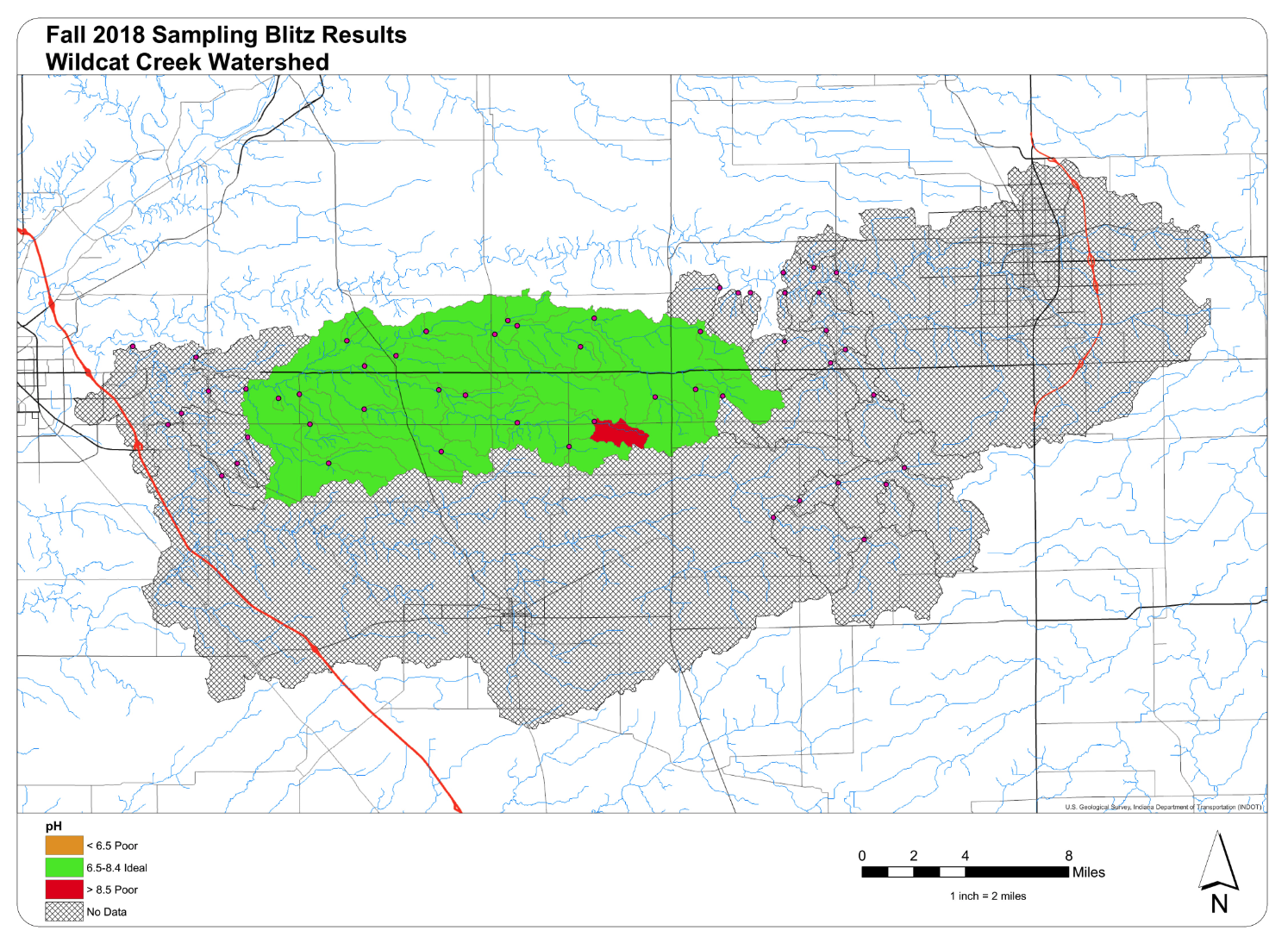
E. coli – E. coli is an indicator organism used to monitor pathogen concentrations with surface waters. E. coli is present in the intestines of all warm-blooded mammals and can survive and reproduce outside of the body. Untreated sewage, combined sewer overflows, polluted discharges, input from animals, and source populations can all contribute E. coli to surface waters. In Indiana, concentrations measuring greater than 235 colonies/100 mL are deemed non-supporting of their designated use. Those watersheds which do not meet water quality standards are shown in red.